How Stem Cells Help Babies with Rare Conditions
Stem cell therapy is offering new hope for newborns with rare, life-threatening conditions. These therapies focus on repairing damaged tissues rather than just managing symptoms, potentially improving outcomes for conditions like bronchopulmonary dysplasia (BPD), hypoxic-ischemic encephalopathy (HIE), and intraventricular hemorrhage (IVH). Here's what you need to know:
- Stem Cells: Found in umbilical cord blood and tissue, these cells can repair and protect at the cellular level.
- Conditions Treated: Early trials show promise for lung damage (BPD), brain injuries (HIE), and brain bleeding (IVH).
- Current Challenges: Traditional treatments often support survival but don't address underlying damage.
- Clinical Trials: Early studies suggest safety and potential benefits, with improved survival and developmental outcomes in some cases.
- Cord Blood Banking: Families can store stem cells at birth for future treatments, offering a genetic match for the child and potential use for siblings.
Stem cell therapy is still in its early stages, but research is advancing rapidly, offering hope for families facing these challenging conditions.
Rare Conditions That Affect Newborns
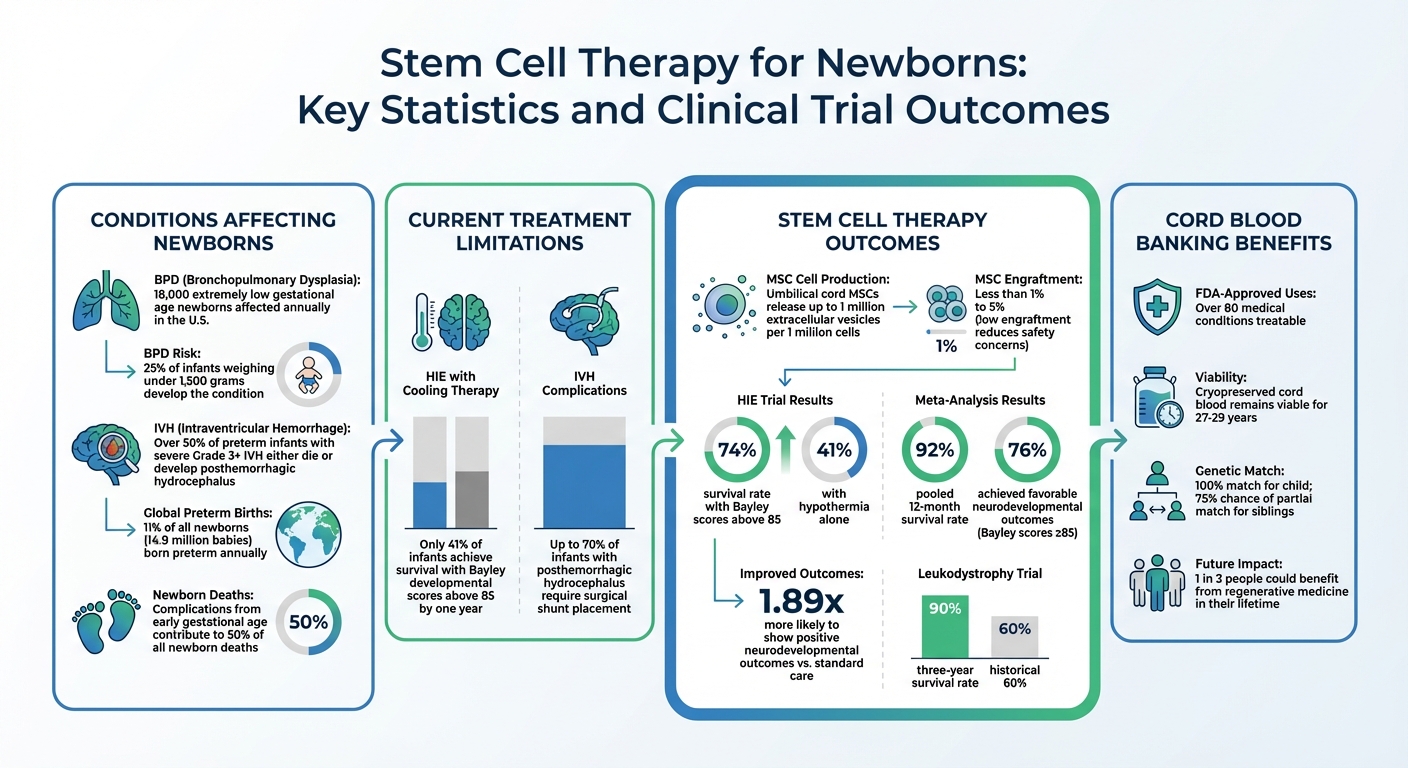
Three rare conditions pose serious challenges for newborns: Bronchopulmonary Dysplasia (BPD), Hypoxic-Ischemic Encephalopathy (HIE), and Intraventricular Hemorrhage (IVH). BPD, a chronic lung disease common among premature infants, affects around 18,000 extremely low gestational age newborns each year in the U.S., with 25% of infants weighing under 1,500 grams developing the condition. HIE results from insufficient oxygen or blood flow to a baby’s brain during or around birth, leading to brain cell damage. IVH, on the other hand, involves bleeding in the brain’s ventricular system, with over 50% of preterm infants experiencing severe (Grade 3 or higher) IVH either succumbing to complications or developing posthemorrhagic hydrocephalus.
Diagnosis for these conditions relies on specific methods: BPD is identified by tracking oxygen and ventilatory needs, HIE through neurological exams and biomarkers, and IVH using cranial ultrasounds. Advances in these diagnostic tools are expected to pave the way for targeted stem cell therapies, bridging the gap between current challenges and potential future treatments.
Why Current Treatments Often Fall Short
Today’s neonatal care focuses on supporting survival rather than repairing damage. For instance, mechanical ventilation helps manage BPD but can also harm fragile lung tissue, sometimes worsening the condition. Treatments like therapeutic cooling for HIE and general supportive measures for IVH are vital but don’t address the underlying tissue injuries.
"Few effective therapies are currently available to ameliorate the injuries resulting from these disorders." - Yun Sil Chang, Department of Pediatrics, Samsung Medical Center
Even with standard cooling therapy, only 41% of infants with HIE achieve survival with Bayley developmental scores above 85 by one year. Similarly, up to 70% of infants with posthemorrhagic hydrocephalus caused by IVH require surgical shunt placement. Neonatology is now pushing the limits of viability at 22 weeks of gestation, a stage where lung and other tissues are especially fragile, highlighting the urgent need for therapies that go beyond life support to actively repair damage.
Effects on Families and Long-Term Health
The effects of these conditions extend well beyond the NICU. Infants with BPD often face lifelong complications like asthma, emphysema, and reduced physical endurance. Survivors of severe IVH may require repeated shunt surgeries, while those with HIE frequently experience cerebral palsy or developmental delays.
For families, the challenges are both emotional and financial. Long NICU stays, multiple surgeries, and ongoing medical care can create overwhelming financial strain. With limited incentives for pharmaceutical companies to develop treatments for such rare conditions, many families are left relying on experimental options. Globally, 11% of all newborns (about 14.9 million babies) are born preterm, with complications from early gestational age contributing to half of all newborn deaths. These staggering numbers highlight the need for regenerative therapies that could ease these burdens and improve outcomes for both infants and their families.
How Stem Cells Treat Neonatal Conditions
Traditional neonatal treatments have largely focused on supporting vital functions rather than repairing damage. Stem cell therapies, however, aim to do more by activating the body's natural repair mechanisms to heal damaged tissue. At the heart of these therapies are Mesenchymal Stromal Cells (MSCs), which don't replace injured cells directly. Instead, they work through a "hit and run" mechanism, releasing therapeutic factors that stimulate repair while minimizing risks like tumor formation.
"In principle, cell therapies have the potential to protect, repair, or sometimes regenerate vital tissues; and improve or sustain organ function."
– Advances in neonatal cell therapies: Proceedings of the First Neonatal Cell Therapies Symposium
Mesenchymal Stromal Cells (MSCs) and Their Role
MSCs primarily heal through paracrine signaling, meaning they release substances that encourage repair rather than integrating into the damaged tissue themselves. These substances, collectively called the secretome, include cytokines, growth factors, and extracellular vesicles (EVs). EVs are particularly important because they carry genetic material and proteins that help reprogram inflammatory cells to promote healing.
When it comes to neonatal care, umbilical cord-derived MSCs from Wharton's jelly are often preferred. These cells are collected non-invasively after birth, making them an ethical and practical choice. They also have a higher capacity for growth and produce stronger anti-inflammatory effects. For example, these cells can release up to 1 million EVs per 1 million cells. Interestingly, their low engraftment rates - typically less than 1% to 5% - are actually beneficial, as they reduce long-term safety concerns while still delivering therapeutic results.
"The absence of long-term adverse effects including tumorigenicity after MSC transplantation might be attributable to the absent engraftment of the transplanted MSCs; thus, they exert their therapeutic function by a 'hit and run' mechanism."
– Yun Sil Chang, Department of Pediatrics, Samsung Medical Center
These characteristics make MSCs a promising tool for addressing neonatal conditions.
Real-World Applications of Stem Cell Therapy in Newborns
Clinical trials are beginning to show how stem cell therapies can make a difference in neonatal care. Early studies have reported positive outcomes for conditions like bronchopulmonary dysplasia (BPD), hypoxic-ischemic encephalopathy (HIE), and intraventricular hemorrhage (IVH):
- In 2014, Dr. Yun Sil Chang and her team at Samsung Medical Center treated 9 preterm infants (average gestational age: 25 weeks) at high risk for BPD. The infants received a single intratracheal dose of allogeneic human umbilical cord blood-derived MSCs - either 10 million or 20 million cells per kilogram - at an average age of 10 days. Follow-ups at 2 years showed no adverse respiratory or neurodevelopmental effects.
- For brain injuries, Dr. C. Michael Cotten from Duke University led a Phase I trial in 2014 involving 23 neonates with HIE. These infants received intravenous infusions of their own umbilical cord blood cells, with doses ranging from 10 to 50 million cells. The results were promising, with a 74% survival rate and Bayley developmental scores above 85 after one year, compared to 41% in a group treated only with hypothermia.
- In 2018, researchers at Samsung Medical Center conducted a Phase I trial for severe Grade 3 or 4 IVH in 9 premature infants. These babies received a single intraventricular dose of allogeneic UCB-MSCs at an average age of 11.6 days. The study reported no dose-limiting toxicities or serious adverse events related to the treatment.
These early trials highlight the potential of stem cell therapies to transform neonatal care, offering hope for conditions that were once considered untreatable.
Clinical Trial Results and Research Findings
Phase 1 Trial Findings
Phase 1 trials have confirmed that stem cell treatments are both safe and practical for newborns. These studies showed that various types of stem cells and delivery methods do not pose risks to vulnerable infants.
In the Netherlands, the PASSIoN trial tested this approach on 10 term newborns with perinatal arterial ischemic stroke. Led by Dr. M. Benders at Utrecht University and completed in 2024, the study used a single intranasal dose of bone marrow-derived MSCs. The results were encouraging - intranasal delivery proved feasible in a neonatal intensive care unit, and only 2 out of 10 infants (20%) developed cerebral palsy at follow-up, a notable improvement compared to historical data.
Meanwhile, in Australia, the Cord-SaFe trial focused on 23 extremely preterm infants born at around 26 weeks' gestation. These infants received an average of 44.4 million autologous cord blood cells per kilogram at approximately 13 days old. Importantly, no serious adverse events were reported.
These findings have laid the groundwork for exploring whether cord blood banking for rare diseases and stem cell treatments can enhance neurodevelopmental outcomes.
Positive Outcomes and Next Steps in Research
With early safety confirmed, researchers have shifted their focus to potential clinical benefits. Recent studies suggest that stem cell therapies may improve outcomes for neonatal brain injuries. A 2025 meta-analysis of neonates with hypoxic-ischemic encephalopathy (HIE) revealed promising results: a pooled 12-month survival rate of 92%, with 76% of the infants achieving favorable neurodevelopmental outcomes (Bayley scores ≥85). Babies treated with stem cell therapy were nearly twice as likely (1.89 times) to show positive neurodevelopmental outcomes compared to those receiving standard care.
"Stem cell therapy appears safe and may improve neurodevelopmental outcomes in neonates with HIE. However, larger, more robust RCTs are needed before universal recommendations can be made." – Child's Nervous System
Looking ahead, research is expanding to larger Phase 2 and 3 trials, such as the i-SToP-CP and Cord-Cell trials. These studies aim to evaluate the clinical effectiveness of these therapies and explore more accessible options, including off-the-shelf allogeneic cell products and cell-free treatments using extracellular vesicles.
Cord Blood Banking and Stem Cell Therapy
What Cord Blood and Tissue Banking Involves
Cord blood and tissue banking is the process of collecting stem cells from the umbilical cord and placenta right after birth. This procedure doesn’t interfere with delivery or delayed cord clamping. Once the cord is clamped, the collection begins, ensuring a safe and non-invasive experience for both the mother and baby.
Cord blood is a rich source of Hematopoietic Stem Cells (HSCs), which are essential for creating blood and supporting the immune system. These cells are approved by the FDA to treat over 80 medical conditions, including childhood leukemia and sickle cell disease. Meanwhile, cord tissue and placental tissue contain Mesenchymal Stem Cells (MSCs), known for their anti-inflammatory and regenerative abilities. MSCs have the potential to develop into bone, cartilage, and muscle cells, and they’re being studied in clinical trials for treating conditions like autism, cerebral palsy, and Type 1 diabetes.
Studies show that cryopreserved cord blood stem cells can stay viable for up to 27 to 29 years. Banking these cells ensures immediate access to a perfect genetic match for your child, eliminating risks like rejection or graft-versus-host disease. For siblings, cord blood offers a convenient option without requiring an exact immune match. This is particularly beneficial for ethnic minorities, who often face difficulties finding suitable donors in public registries. By storing these cells, families preserve the potential for future treatments, including therapies for rare neonatal disorders.
How Americord Registry Helps Families
With the growing importance of stem cells in medical treatments, finding a reliable banking service is key. Americord Registry provides specialized preservation services, including cord blood, cord tissue, placental tissue, and exosome banking. They use their exclusive CryoMaxx™ Processing method to maximize cell retention, ensuring that the widest range of treatment options remains available for your family. This method preserves not only stem cells but also growth factors and cytokines critical for regenerative therapies.
Americord stores samples in an AABB-accredited, FDA-registered facility equipped with 24/7 monitoring. Their pricing is straightforward, with no rising annual fees. They offer 20-year prepaid plans and even lifetime storage options. To back their services, Americord includes a $110,000 engraftment guarantee, which helps locate an alternative stem cell source if a transplant doesn’t engraft successfully.
One real-life example of their impact is the story of Eli, a 10-year-old boy born with Sickle Cell Disease. In 2020, Eli received a successful transplant using cord blood banked through Americord’s program by his younger brother Gus. Just three years after storing the cells, Eli’s doctors reported he was thriving and showing excellent progress post-transplant.
Conclusion
Stem cell therapies are reshaping neonatal care by focusing on repairing damaged tissues and restoring normal function, rather than merely addressing symptoms. With research indicating that 1 in 3 people could benefit from regenerative medicine during their lifetime, collecting cord blood and tissue at birth becomes a once-in-a-lifetime opportunity to access these potentially life-saving treatments.
Dr. Paul Szabolcs's trial at UPMC Children's Hospital highlights the promise of early stem cell intervention. The study reported a 90% three-year survival rate in leukodystrophy patients, a significant improvement over the historical 60%. Additionally, most children with metabolic disorders in the trial achieved normalized enzyme levels within a year, halting neurological decline. These findings underscore the transformative impact of stem cell therapies.
"Banking my baby's cord blood is like an insurance. I'd feel guilty if I'd passed up such a simple, yet potentially life-saving opportunity." - Catherine W., Mother
Cord blood and tissue collected at birth offer a 100% genetic match for the child and a 75% chance of at least a partial match for siblings. To support families in preserving this vital resource, Americord Registry provides advanced storage solutions. Their CryoMaxx™ Processing method, AABB-accredited facilities, and clear, fixed annual pricing ensure a secure and straightforward preservation process.
With FDA-approved treatments for over 80 conditions and ongoing trials exploring applications for cerebral palsy and autism, early stem cell banking offers a powerful way to prepare for future medical advancements.
FAQs
Is stem cell therapy available for babies now or only in trials?
Stem cell therapy for infants is already making strides, with certain treatments - like FDA-approved cord blood therapies - actively being used. Meanwhile, researchers and clinical trials are working to expand its potential, particularly in treating rare conditions that affect newborns.
What risks or side effects could stem cell treatments have for newborns?
Stem cell treatments for newborns come with potential risks that shouldn't be overlooked. These can include infections, tumor formation, and immune system reactions. When donor-derived cells are used, complications like immune rejection or graft-versus-host disease may arise, posing serious health challenges. Additionally, some treatments - especially those not properly regulated - might lead to issues such as neurological changes or heart problems.
To ensure safety, it’s critical that such therapies are carried out exclusively in well-regulated clinical environments where strict protocols and oversight are in place.
Should I bank cord blood and tissue in case my baby needs it later?
Banking cord blood and tissue can provide a resource with potential lifesaving benefits. Stem cells collected from these sources have already been used to treat conditions such as sickle cell disease, autism, and various genetic disorders. By preserving these cells at birth, families gain access to a valuable option in regenerative medicine that could address future medical challenges.
The views, statements, and pricing expressed are deemed reliable as of the published date. Articles may not reflect current pricing, offerings, or recent innovations.




