Advances in Muscle Regeneration Using Cord Blood
Advances in Muscle Regeneration Using Cord Blood
Umbilical cord blood is emerging as a powerful tool for repairing damaged skeletal muscle. Unlike traditional treatments, cord blood stem cells don’t just replace damaged tissue - they send signals that trigger the body’s natural repair processes. This process reduces inflammation, activates muscle repair cells (satellite cells), and protects against further damage. Key highlights include:
- How It Works: Cord blood stem cells use paracrine signaling to release growth factors and cytokines that aid muscle healing.
- Inflammation Control: They shift immune cells to a healing state, reducing harmful inflammation.
- Satellite Cell Activation: Cord blood triggers muscle repair cells to grow and rebuild damaged tissue.
- Protection Against Damage: These stem cells prevent muscle degradation by reducing oxidative stress and stopping protein breakdown.
- Delivery Methods: Intramuscular injections target specific sites, while intravenous delivery supports systemic repair. Exosomes, a cell-free option, show promise for safer and more efficient treatments.
Research has demonstrated success in improving muscle mass, strength, and recovery in animal models of aging and injury. Cord blood banking ensures families can store these cells for future therapies, offering a ready source for regenerative medicine.
How Cord Blood Helps Repair Muscle Tissue
Cord blood stem cells play a critical role in supporting the body's natural ability to repair muscle tissue. They act as signaling agents, releasing a mix of growth factors, chemokines, and cytokines - either as soluble proteins or within extracellular vesicles. This process, known as paracrine signaling, allows these cells to communicate with surrounding tissues, triggering healing mechanisms without directly transforming into muscle fibers. This signaling helps reduce inflammation and activates the body's natural repair systems.
Reducing Inflammation and Regulating Immune Response
One of the key roles of cord blood stem cells is managing inflammation during muscle repair. They achieve this by influencing macrophages - immune cells that play a role in healing. Specifically, they shift macrophages from a pro-inflammatory state (M1 phenotype) to a pro-repair state (M2 phenotype). While some inflammation is necessary for healing, prolonged or excessive inflammation can lead to muscle damage, fatigue, and fibrosis. Cord blood–derived mesenchymal stromal cells (MSCs) also release VEGF, a factor that aids tissue restoration. Their ability to modulate immune responses without significant rejection makes them particularly effective.
Activating Muscle Satellite Cells
Satellite cells are the muscle's natural repair system, and cord blood stem cells help activate them. These stem cells release paracrine factors such as Macrophage Inflammatory Protein 2 (MIP-2), Monocyte Chemotactic Protein 1 (MCP-1), and Activin A, which stimulate the PI3K/Akt signaling pathway. This pathway is essential for muscle growth and protection against atrophy. Through this process, satellite cells transition from a resting state (Pax7-positive) to an active, proliferative state (MyoD-positive), eventually forming myoblasts that repair damaged muscle fibers.
In a 2016 study by CHA University, researchers tested this process using a rat model with induced muscle atrophy. They injected human umbilical cord mesenchymal stem cell–derived conditioned medium (UC-CM) into the soleus muscles of the rats. The results were impressive: muscle mass and fiber size significantly improved, with a 2.3-fold decrease in MuRF-1 and a 2.1-fold decrease in MAFbx expression - both markers of muscle degradation.
Preventing Muscle Cell Damage
Cord blood stem cells do more than just initiate repair; they also protect muscle tissue from further damage. They achieve this through several mechanisms:
- Anti-Apoptotic Activity: By transferring microRNAs like let-7-5p and anti-apoptotic proteins, they help prevent programmed cell death in damaged muscle cells.
- Reducing Oxidative Stress: They downregulate NOX2 (a source of oxidative stress) and upregulate NRF2/ARE pathways, which protect cells from oxidative damage.
- Inhibiting Muscle Atrophy: By suppressing MuRF-1 and MAFbx ligases, they prevent muscle protein breakdown and mass loss.
- Promoting Survival Signaling: Activation of the PI3K/Akt pathway supports cellular recovery and stability.
| Mechanism | Biological Action | Protective Outcome |
|---|---|---|
| Anti-Apoptotic | Transfer of microRNA (let-7-5p) and anti-apoptotic proteins | Prevents programmed cell death in damaged muscle tissue |
| Anti-Oxidative | Downregulation of NOX2; upregulation of NRF2/ARE | Reduces oxidative stress and cellular harm |
| Atrophy Suppression | Inhibition of MuRF-1 and MAFbx ligases | Prevents muscle protein degradation and mass loss |
| Survival Signaling | Activation of the PI3K/Akt pathway | Promotes cellular recovery and functional stability |
Animal studies have shown that injecting UC-MSC conditioned medium into atrophied muscles led to a twofold improvement in muscle mass and fiber size compared to untreated controls. These protective actions highlight the potential of cord blood stem cells to not only repair but also shield muscle tissue from further harm after an injury.
Laboratory Studies on Cord Blood and Muscle Regeneration
Research with animal models has shown that cord blood stem cells can help reverse muscle deterioration in various situations. Mice and rats have been central to these studies, offering insights into how cord blood impacts damaged or aging muscle.
Treating Age-Related Muscle Loss
In May 2023, a team led by Chao Wang tested clinical-grade human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) on SAMP8 mice, which naturally develop age-related muscle loss similar to humans. They also included a group of mice with induced aging using D-galactose. The results were promising: hUC-MSCs improved grip strength and reduced fatigue by activating satellite cells and boosting autophagy. The researchers highlighted these findings:
"hUC-MSCs significantly restored skeletal muscle strength and performance in both mouse models via mechanisms including raising the expression of crucial extracellular matrix proteins, activating satellite cells, enhancing autophagy, and impeding cellular aging." - Chao Wang et al.
Later, in July 2025, a study published in Stem Cell Research & Therapy explored a different approach using exosomes - tiny vesicles secreted by cord blood stem cells. Researchers injected these exosomes into 24-week-old SAMP10 mice over 12 weeks. The outcomes included a 37.6% increase in gastrocnemius muscle weight and a 30.2% increase in soleus muscle weight. The mice also showed a 21.3% improvement in grip strength and a 17.3% boost in running endurance. Additionally, the treatment reduced mitochondrial injury by 26.6% and cut lipid droplet accumulation by 25.3%.
Cord blood therapies have also shown potential in addressing muscle atrophy caused by disuse.
Muscle Recovery After Injury and Disuse
Cord blood treatments don’t just benefit age-related muscle loss - they also aid recovery from disuse-induced atrophy. This type of muscle loss, often caused by prolonged bed rest or immobilization, presents unique challenges. In October 2016, researchers used a rat model to simulate disuse atrophy through two weeks of hindlimb suspension. They then injected human umbilical cord mesenchymal stem cell-derived conditioned medium (UC-CM) into the soleus muscle. The treatment reduced atrophy-related ubiquitin E3-ligases and lactate buildup. As noted in Tissue and Cell:
"UC-CM provides an effective stimulus to recover muscle status and function in atrophied muscles." - Tissue and Cell, 2016
These studies reveal that cord blood therapies - whether as whole cells, exosomes, or conditioned medium - can restore muscle function after both age-related decline and atrophy caused by disuse, using a variety of mechanisms.
Methods for Delivering Cord Blood Treatments
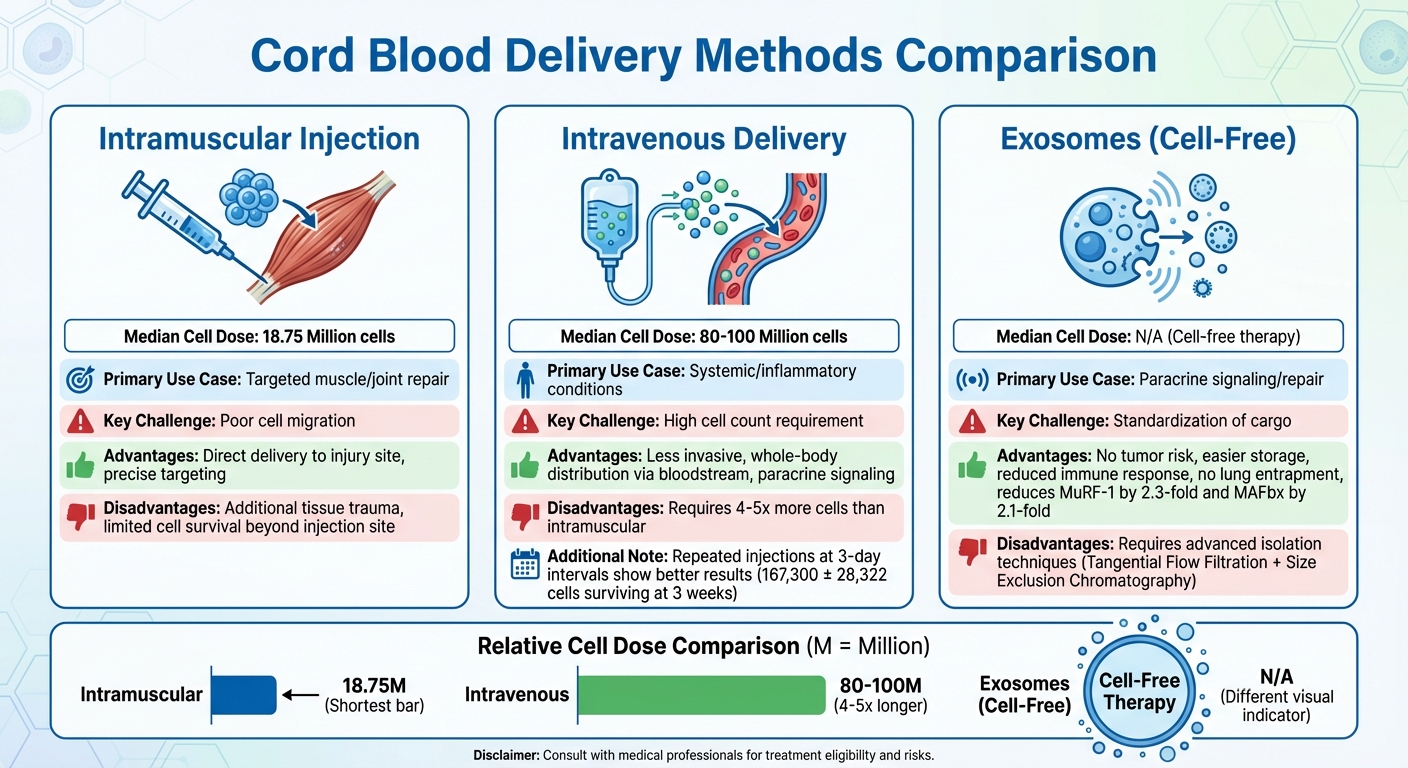
Cord Blood Stem Cell Delivery Methods for Muscle Regeneration
Delivering cord blood stem cells to damaged muscle tissue requires careful consideration of the delivery method. Researchers have explored several approaches, each with its own strengths and challenges.
Comparing Injection Methods: Muscle vs. Vein
Intramuscular injections involve delivering cells directly to the site of injury. This targeted approach ensures the cells are placed where they’re needed most. However, it can also cause additional trauma to the tissue, and the cells may struggle to survive or migrate beyond the injection site. Clinical trials using this method typically administer a median dose of 18.75 million cells per treatment.
Intravenous delivery, on the other hand, is the most commonly used method in clinical settings. It’s less invasive and allows the cells to travel throughout the body via the bloodstream. This method works primarily through paracrine signaling, where the cells release growth factors and cytokines to stimulate repair mechanisms in distant tissues. However, it requires a much higher number of cells, with clinical trials typically using a median dose of 80 to 100 million cells per treatment. A study conducted by the Affiliated Hospital of Southwest Medical University in May 2018 found that repeated intravenous injections at 3-day intervals led to better cell survival (167,300 ± 28,322 cells at 3 weeks) and improved functional recovery compared to administering a single large dose.
| Delivery Method | Median Cell Dose | Primary Use Case | Key Challenge |
|---|---|---|---|
| Intramuscular | 18.75 Million | Targeted muscle/joint repair | Poor cell migration |
| Intravenous | 80–100 Million | Systemic/inflammatory conditions | High cell count requirement |
| Exosomes | N/A (Cell-free) | Paracrine signaling/repair | Standardization of cargo |
These methods highlight the potential for alternative strategies, including cell-free approaches, to enhance treatment outcomes.
Using Exosomes to Improve Treatment Results
While direct cell injections remain widely used, exosomes offer a cutting-edge alternative that complements these methods. Exosomes are tiny vesicles released by cord blood stem cells, carrying mRNA, miRNA, and proteins directly to damaged tissues. They provide several advantages over whole-cell therapy, including no risk of tumor formation, easier storage, and reduced immune response.
"MSC-EVs could be advantageous compared with the parental cells because of their specific cargo containing mRNAs, miRNAs, and proteins that can be biologically transferred to recipient cells." - Valentina Saccone, Researcher
Exosomes can activate satellite cells and suppress muscle-wasting enzymes. Research shows they reduce atrophy-related ligases MuRF-1 and MAFbx by 2.3-fold and 2.1-fold, respectively. Unlike whole cells, exosomes lack a nucleus, so they cannot replicate, eliminating the risk of becoming trapped in lung microvasculature - a common concern with systemic cell injections.
Scaling Treatments and Maintaining Ethical Standards
As these delivery methods evolve, scaling cord blood therapies for broader clinical use brings both practical and ethical considerations. One of the key advantages of cord blood is its availability - it’s often discarded as medical waste after birth, making it a non-controversial source of cells. Existing cord blood banks also provide the infrastructure needed for "off-the-shelf" treatments, avoiding the need for patient-specific cell generation.
To meet the high cell counts required for intravenous treatments, researchers are leveraging the impressive self-renewal capacity of umbilical cord stem cells, which can undergo up to 300 cell divisions with a doubling time of just 30 to 36 hours. Advanced isolation techniques, such as Tangential Flow Filtration combined with Size Exclusion Chromatography, are being developed to produce high-purity exosomes for clinical use. Cell-free therapies like exosomes simplify the scaling process while prioritizing safety and efficacy.
Why Cord Blood Banking Matters for Muscle Regeneration
Future Medical Options Through Cord Blood Storage
Cord blood banking offers a forward-looking approach to regenerative medicine, particularly for muscle repair. By preserving umbilical cord blood and tissue at birth, families secure a source of stem cells that can remain viable for decades. These cells stand out due to their longer telomeres and higher ability to replicate compared to adult stem cells, making them a strong candidate for treating chronic degenerative conditions.
For example, umbilical cord tissue mesenchymal stem cells (MSCs) activate 2,010 myogenic genes in just one week - far exceeding the 326 genes activated by cord blood MSCs. This process is largely driven by CD90+ cells, which are essential for muscle cell development. In studies involving muscular dystrophy in animal models, a single injection of cord blood CD34+ cells led to the appearance of human dystrophin-positive muscle fibers 45 days after treatment.
"Cells of fetal origin, from cord blood to placenta and amniotic fluid, can be easily obtained without ethical concern, expanded and differentiated in culture, and possess immune-modulatory properties." - Michela Pozzobon, Fondazione Istituto di Ricerca Pediatrica Città della Speranza
Another advantage of fetal stem cells is their immune-privileged status, which lowers the likelihood of rejection or complications like graft-versus-host disease. This makes them a safer option compared to adult-derived stem cells. Considering that muscle makes up over 40% of the human body, access to these reparative cells could provide meaningful, long-term therapeutic benefits.
How Americord Registry Supports Regenerative Medicine
To make these advancements practical, proper cord blood preservation is key. Americord Registry offers a range of storage services, including cord blood, cord tissue, placental tissue, and newborn exosome banking, all designed to support cutting-edge muscle regeneration treatments.
Their exosome banking service, for instance, focuses on storing cell-free vesicles that can suppress muscle-wasting enzymes and stimulate satellite cells. This approach avoids the challenges associated with whole-cell transplants while still promoting muscle repair. Using CryoMaxx™ Processing and 5-compartment storage vials, Americord ensures the preserved materials meet the stringent standards required for clinical applications.
With AABB accreditation and FDA-approved methods, Americord provides the reliability needed for future therapies. As research increasingly explores multicomponent storage - where preserved materials can be used to create platelet gel, plasma, and other regenerative products - having a variety of tissue types stored expands the possibilities for treatment options down the road.
Conclusion and Future Research Directions
Main Findings from Recent Research
Recent studies highlight that umbilical cord tissue (UCT) offers greater potential for muscle regeneration compared to cord blood. Cells derived from UCT not only double their proliferation rate but also show reduced signs of aging. A key distinction lies in CD90 expression: UCT cells are marked as CD105⁺CD90⁺, while cord blood cells are CD105⁺CD90⁻. This difference allows UCT cells to create strong, longitudinal muscle fibers, unlike the less efficient "myosacs" formed by cord blood cells.
An exciting alternative to direct cell transplantation is the secretome approach. The secretome - comprising proteins and factors secreted by umbilical cord cells - has shown the ability to suppress muscle-wasting enzymes and activate the PI3K/Akt pathway, which promotes muscle growth. A clinical study involving 39 women with stress urinary incontinence demonstrated significant improvements within three months after treatment with approximately 430 million cord blood stem cells.
Animal studies also provide promising insights. Transplanting as few as 900 muscle stem cells improved muscle function in disease models, suggesting that with proper preservation, cord blood and tissue could support multiple treatments over a person’s lifetime. These findings emphasize the importance of refining preservation techniques and exploring broader applications.
The Need for Continued Cord Blood Research
Despite these advancements, challenges persist. Alarmingly, nearly 90% of donated cord blood is discarded due to insufficient cell counts. Exploring multicomponent cord blood banking - which repurposes unused units into platelets, plasma, and other regenerative products - could transform this inefficiency into a valuable resource.
"Large multicenter randomized trials with standardized protocols and long-term follow-up... are needed to confirm durable benefit and enable routine clinical integration."
- PubMed Review
One of the most pressing technical hurdles is maintaining cell potency during in vitro expansion. Muscle stem cells tend to lose their regenerative properties under traditional lab conditions, limiting their therapeutic potential. Developing better pharmacological modulators and biophysical environments is crucial to preserving their functionality.
Additionally, the lack of standardization across the field - spanning cell sources, manufacturing processes, dosages, and delivery methods - creates inconsistencies that hinder clinical adoption. Addressing these gaps through continued research and investment is essential. By doing so, cord blood therapies could become a reliable option for treating muscle injuries, age-related muscle decline, and genetic disorders, paving the way for more routine clinical use in the future.
FAQs
How do stem cells from cord blood help with muscle repair and reduce inflammation?
Cord blood stem cells play a key role in muscle repair by releasing bioactive molecules that help manage inflammation and support tissue healing. These molecules work by adjusting the immune system, reducing damaging inflammation, and encouraging the body’s natural repair processes.
Studies indicate that cord blood stem cells can assist in rebuilding damaged muscle tissue and enhancing recovery following injuries. This positions them as a promising option in the field of regenerative medicine.
What makes exosomes a better option than whole-cell therapies for muscle regeneration?
Exosomes offer a compelling option for muscle regeneration therapies. These tiny vesicles deliver bioactive molecules - including proteins, RNA, and lipids - that actively support tissue repair and healing.
What sets exosomes apart from whole-cell therapies is their reduced risk of complications. Unlike living cell transplants, exosomes are less likely to trigger immune rejection or lead to tumor development, making them a safer alternative.
By leveraging the regenerative abilities of exosomes, researchers aim to enhance muscle recovery without the hurdles tied to traditional cell-based treatments. This positions exosomes as a promising tool in the advancement of regenerative medicine.
How does cord blood banking support future muscle regeneration therapies?
Cord blood banking is making strides in muscle regeneration therapies by offering a reliable source of mesenchymal stem cells (MSCs). These cells, especially those sourced from umbilical cord tissue, have shown an impressive ability to develop into muscle tissue. Studies reveal that MSCs from cord tissue stand out due to their higher purity, enhanced ability to differentiate, and slower aging process, making them a strong candidate for treatments aimed at repairing skeletal muscle.
Storing cord blood and tissue provides access to powerful, fetal-derived stem cells with immune-modulating properties. These cells can be expanded and customized for therapeutic purposes, offering new possibilities for addressing muscle injuries and diseases where the body’s natural stem cells fall short. By choosing to bank cord blood, families secure a resource that aligns with advancements in regenerative medicine, opening doors to promising muscle repair therapies.
The views, statements, and pricing expressed are deemed reliable as of the published date. Articles may not reflect current pricing, offerings, or recent innovations.