Umbilical Cord Blood in Rare Genetic Disorder Trials
Umbilical cord blood is transforming the treatment of rare genetic disorders, offering new hope for families. This blood, collected at birth, contains hematopoietic stem cells (HSCs), which can develop into various blood cell types. These stem cells are now being used in clinical trials to treat conditions like sickle cell disease, thalassemia, Hunter syndrome, and Krabbe disease.
Key highlights:
- Lower rejection risks: Cord blood tolerates mismatched immune markers better than bone marrow, making it accessible to more patients.
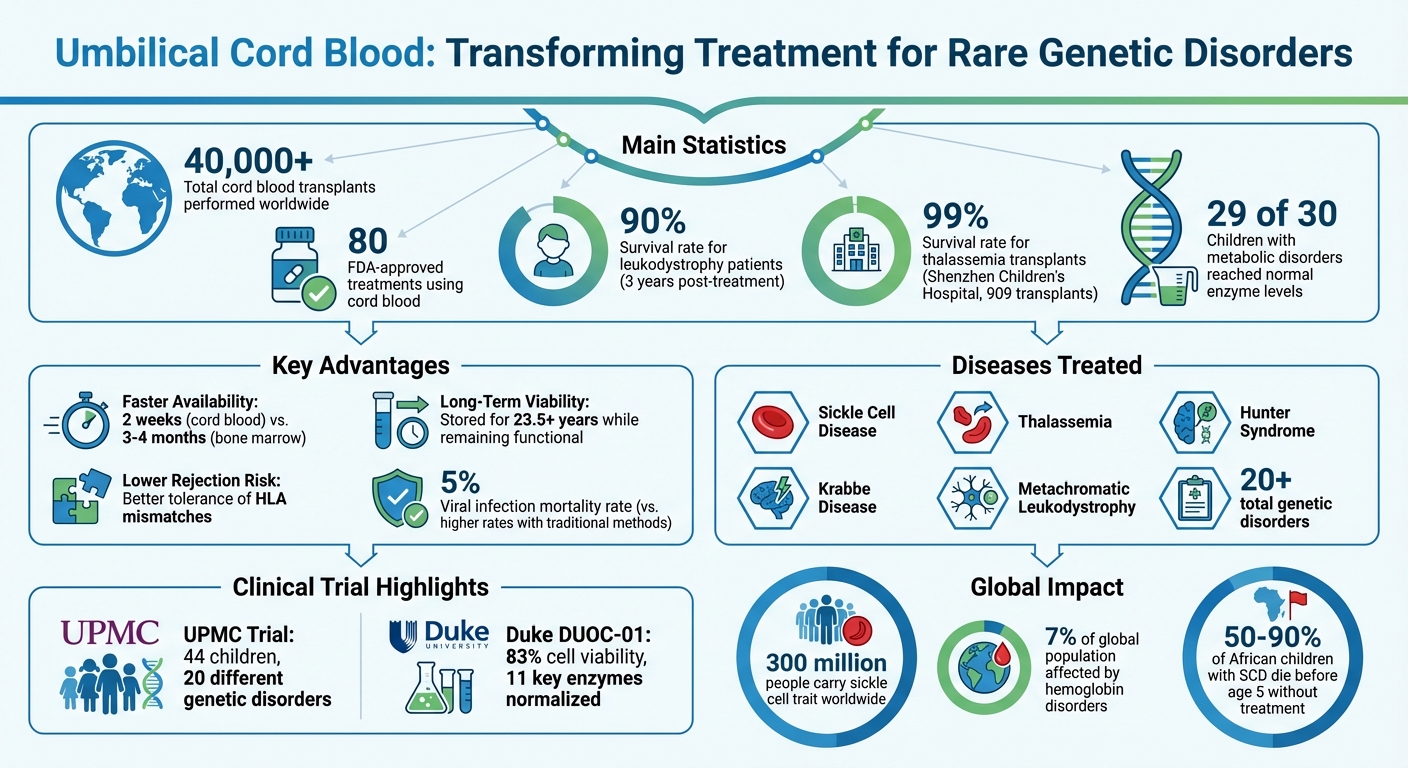
- Success in trials: A 2020 study showed 90% survival rates for leukodystrophy patients and normalized enzyme levels in 29 of 30 metabolic disorder cases.
- Faster availability: Cord blood units can be ready in 2 weeks, compared to months for bone marrow donors.
- Banking benefits: Stored cord blood remains viable for over 23.5 years, providing a long-term health resource for families.
With over 40,000 transplants performed and 80 FDA-approved treatments, cord blood is proving to be a vital tool in modern medicine. Families can secure future treatment options by banking cord blood at birth.
Rare Genetic Disorders Being Treated with Cord Blood
Cord blood transplants are showing promise as a treatment for genetic disorders that previously had limited options. These disorders typically fall into two groups: blood disorders affecting hemoglobin production and metabolic disorders caused by enzyme deficiencies. Encouraging trial results have led researchers to apply cord blood transplants to specific rare conditions.
Sickle Cell Disease and Thalassemia
Sickle Cell Disease (SCD) and Thalassemia are hemoglobin disorders affecting up to 7% of the global population. Around 300 million people worldwide carry the sickle cell trait, and in Africa, 50% to 90% of children born with SCD die before reaching age five.
SCD causes misshapen blood cells that block vessels, leading to painful crises, strokes, and organ damage. Thalassemia patients often require frequent blood transfusions, which can cause iron overload and further harm organs.
Cord blood transplants offer a potential cure by replacing defective blood-forming cells with healthy donor cells. For example, a protocol developed at UPMC Children's Hospital uses low-dose chemotherapy and immunosuppressants to help donor cells engraft successfully, even without a perfect immune match. This is especially beneficial for ethnic minorities who often face difficulties finding compatible bone marrow donors. In China, Shenzhen Children's Hospital reported performing 909 thalassemia transplants over 11 years, achieving an impressive 99% survival rate, thanks to the country's extensive public vs private cord blood banking options.
Hurler Syndrome and Krabbe Disease
Unlike hemoglobin disorders, metabolic conditions like Hurler Syndrome and Krabbe Disease result from enzyme deficiencies. These missing enzymes are essential for breaking down harmful substances. Without them, toxins build up in cells, causing severe brain damage and neurological decline. If untreated, these diseases are often fatal within a few years of onset.
Cord blood transplants provide donor stem cells that produce the missing enzymes, stopping toxin accumulation. This allows many children to regain lost developmental skills.
Timing is critical for fast-progressing diseases like Krabbe. In December 2023, Dr. Paul Orchard from the University of Minnesota treated seven-month-old Lyla Edgington, diagnosed with Hurler Syndrome (MPS Type 1), using a perfectly matched cord blood unit. The 25-minute transfusion halted brain damage, and Lyla has shown no signs of the disorder in the years since. These targeted treatments highlight the role of cord blood banking in expanding options for rare genetic conditions.
Recent Clinical Trials Using Cord Blood
Two groundbreaking studies have highlighted the potential of cord blood in treating rare genetic disorders in children. With distinct methods, both trials have delivered promising outcomes, offering new hope for affected families.
UPMC Children's Hospital Pittsburgh Trial
In July 2020, Dr. Paul Szabolcs and his team at UPMC Children's Hospital shared results from a study involving 44 children diagnosed with 20 different genetic disorders. This included conditions like sickle cell disease, thalassemia, Hunter syndrome, Krabbe disease, and metachromatic leukodystrophy.
The team implemented a reduced-intensity conditioning (RIC) regimen, which combined low-dose chemotherapy and immunosuppressants delivered intravenously. This strategy aimed to suppress the immune system enough to prevent rejection without causing harm to essential organs.
"We approached the topic with the mindset to design a regimen that carefully balances low-intensity chemo (bringing safety) with sufficiently effective immunotherapy to blast away the patients' immune system, therefore preventing rejection", explained Dr. Szabolcs.
A standout feature of the trial was the introduction of a "plug-in" immune boost. A small, refrozen portion of the cord blood unit was infused weeks after the initial transplant to aid immune recovery.
The results, published in Blood Advances, were encouraging: 90% of symptomatic leukodystrophy patients were alive three years post-treatment, with a viral infection mortality rate of just 5%. Additionally, no patients experienced severe chronic graft-versus-host disease. These advancements pave the way for future therapies that build on this foundation.
Duke University Trial with DUOC-01
At Duke University Medical Center, researchers developed DUOC-01, a therapy derived from 20% of a banked cord blood unit, while the remaining 80% was used for the patient’s primary transplant. Over 21 days, the cord blood cells were cultured to develop into macrophage or microglia-like cells capable of producing critical enzymes.
DUOC-01 addresses an essential gap in care by delivering functional enzymes directly to the central nervous system. Approximately one month after the initial transplant, DUOC-01 is administered via lumbar puncture into the spinal fluid. This timing ensures that the therapy supports the brain during its most vulnerable early recovery period.
"DUOC-01 is intended to be delivered intrathecally after systemic transplantation and after engraftment of donor cells as a bridging therapy to provide functional enzyme and perhaps other beneficial products to the brain in the early post-transplant period", explained Dr. Joanne Kurtzberg, who leads this research.
The Phase 1 clinical trial (NCT02254863) focused on patients aged 1 week to 22 years with inherited metabolic disorders, including Krabbe disease, Hunter syndrome, adrenoleukodystrophy, and metachromatic leukodystrophy. Preclinical studies showed that DUOC-01 cells demonstrated normal activity for 11 key lysosomal enzymes, with an average viability of 83% after the 21-day culture period.
What These Trials Mean for Cord Blood Banking
Benefits of Cord Blood Banking for Families
Clinical trials continue to highlight the importance of cord blood preservation, showcasing why it can be a lifeline for families. One of the biggest advantages? Cord blood is ready when you need it. Unlike bone marrow or peripheral donors, which can take 3–4 months to locate and prepare, banked cord blood is frozen and available within just two weeks. For families dealing with rare genetic disorders, that time difference could be life-changing.
Another key benefit is the less restrictive HLA matching required for cord blood. This makes it a more accessible option, particularly for ethnic minorities who often face challenges finding donor matches. As Dr. Paul Szabolcs put it, "The probability of a perfect match is very low, but with a cord blood graft, we have a chance to overcome this discrepancy over the course of a couple months."
Recent trials have also underscored cord blood's potential in treating over 20 rare diseases, including sickle cell disease, thalassemia, Hunter syndrome, and Krabbe disease. For example, a UPMC trial showed that 29 out of 30 children with metabolic disorders reached normal enzyme levels within a year and experienced a complete stop in neurological decline. What’s more, cord blood can be stored for over 20 years while retaining functional stem cells, giving families long-term treatment options. These benefits make choosing a reliable cord blood banking service a critical decision.
How Americord Registry Supports Treatment Access
Americord Registry builds on these advantages by offering advanced preservation technologies to ensure families have access to future treatment options. Their CryoMaxx™ Processing technology and 5-compartment storage vials are specifically designed to keep cord blood units viable for emerging regenerative medicine applications.
Families can tailor their banking plans to their needs. Options range from the Essential Family Plan, which focuses on storing cord blood, to the Maximum Family Plan, which includes cord blood, cord tissue, placental tissue, and even newborn and maternal exosome banking. This flexibility allows families to choose the level of preservation that works best for them. Americord Registry’s AABB accreditation guarantees that stored units meet the high-quality standards necessary for clinical use.
Additionally, Americord offers access to "Expanded Access" or "Compassionate Use" programs. These programs allow families to use stored units for treatments before full FDA approval, a critical option for addressing rare genetic conditions where approved therapies may still be years away.
With advances in stem cell multiplication technologies, privately banked cord blood is becoming even more valuable for both personal (autologous) use and experimental regenerative treatments. Americord’s straightforward pricing and dedicated customer support make it easier for families to explore these possibilities as stem cell research continues to open new doors for treatment.
Conclusion
Main Points
Clinical trials have confirmed that cord blood is both effective and safe in treating over 20 rare genetic disorders, such as sickle cell disease, thalassemia, Hunter syndrome, and Krabbe disease. These studies showcased impressive success rates and improved safety. As Dr. Paul Szabolcs explained:
"We designed an approach now proven to be efficacious for at least 20 diseases. And we believe it might be effective for many, many more."
One of cord blood's key advantages is its ability to tolerate HLA mismatches, which significantly broadens treatment possibilities - particularly for ethnic minorities who may face challenges finding matched donors. These findings set the stage for even more advancements in the field.
What Comes Next
Early intervention through newborn screening plays a vital role in achieving the best outcomes, as it allows treatment to begin before serious organ or neurological damage occurs. Researchers are now exploring ways to adapt these protocols for adult patients, expanding beyond the traditional focus on pediatric transplants.
As clinical trials continue to reshape treatment approaches, the importance of early intervention and cord blood banking grows. With cord blood remaining viable for over 27 years, stored units offer long-term possibilities for emerging therapies. Americord Registry, with its AABB-accredited facilities and multi-compartment storage systems, provides families with the tools to access these treatments as research progresses.
Cord blood banking isn't just about addressing current medical needs - it’s about planning for the future of regenerative medicine that could potentially impact 1 in 3 people over the course of their lives.
FAQs
Who can use cord blood in these genetic disorder trials?
Cord blood is often used in clinical trials for children diagnosed with rare, non-cancerous genetic conditions such as sickle cell disease, thalassemia, Hunter syndrome, Krabbe disease, and metachromatic leukodystrophy. Participation in these trials depends on specific medical criteria and the availability of matching cord blood units. Typically, these trials are organized through specialized medical centers as part of research focused on assessing both the safety and effectiveness of treatments.
How do doctors decide if cord blood or a donor match is needed?
Doctors consider several factors when deciding on a treatment approach, including donor availability, HLA compatibility, and the patient’s unique medical requirements. Cord blood is frequently selected when a fully HLA-matched donor cannot be found. This is because it’s easier to obtain and carries a reduced risk of graft-versus-host disease (GVHD), particularly in pediatric cases. Additionally, clinical trials have demonstrated its safety and effectiveness in treating rare genetic disorders, especially when donor options are limited.
When should parents bank cord blood for maximum benefit?
Parents might want to consider storing their child’s umbilical cord blood at birth to preserve the stem cells for future medical needs. Cord blood is rich in stem cells that can be used to treat certain genetic and blood-related disorders. Storing it early ensures these cells are readily available for treatments or experimental therapies, particularly for families with a history of genetic conditions. It also provides a flexible cell source for potential medical advancements.
The views, statements, and pricing expressed are deemed reliable as of the published date. Articles may not reflect current pricing, offerings, or recent innovations.